Welcome to the Krell laboratory.
The Krell laboratory is part of the research group “Environmental Microbiology and Biodegradation”. The laboratory is located at the Estación Experimental del Zaidín in Granada (Spain) which is part of the Spanish National Research Council (CSIC). The Krell laboratory participates in the Master “Investigation and Advances in Microbiology” (https://masteres.ugr.es/microbiologia/) at Granada University.
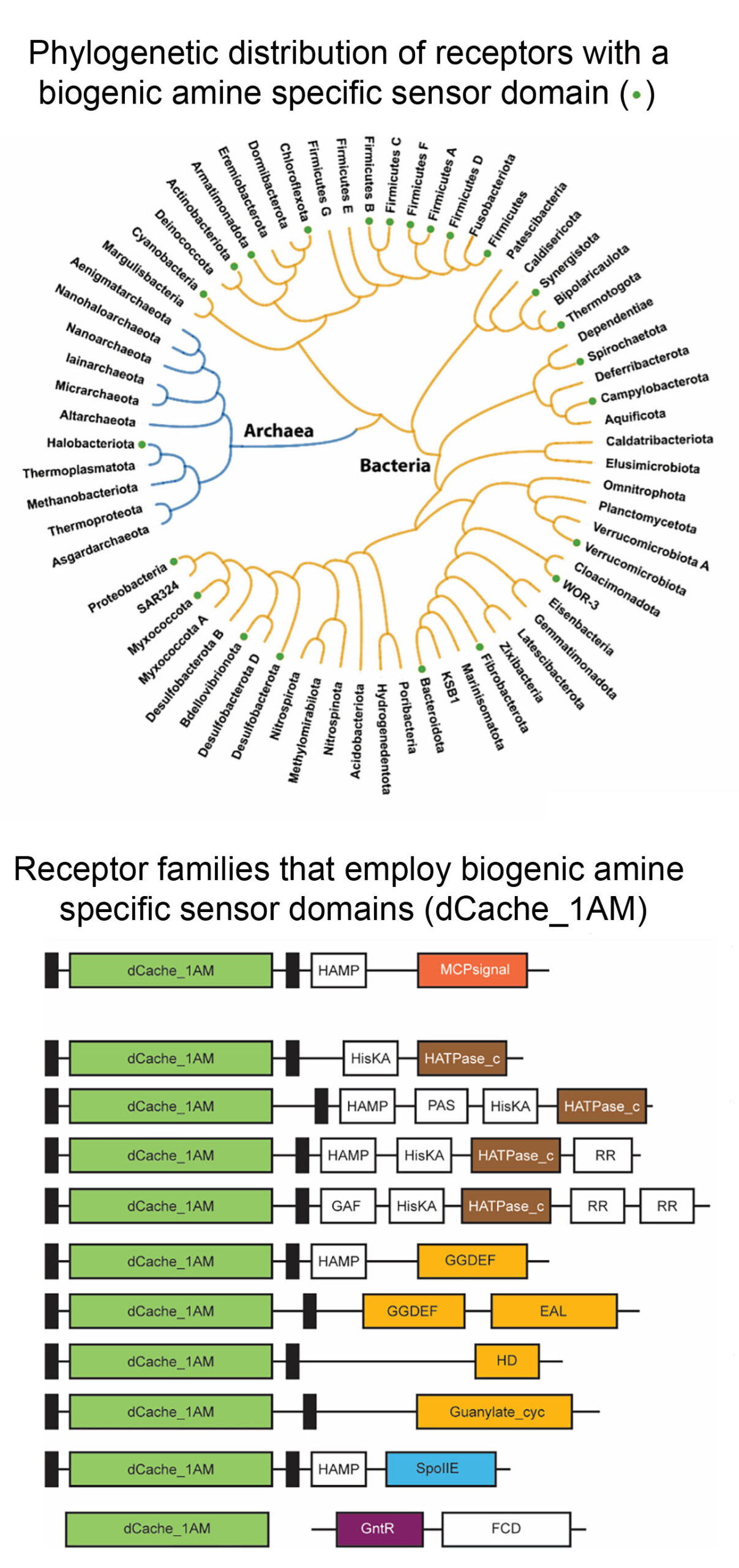
Many bacterial receptors contain sensor domains that bind biogenic amines
Cerna-Vargas et al. (2023) Proc Natl Acad Sci USA 120:e2305837120
Following an analogous approach that we have used to identify amino acid specific sensor domains (Gumerov et al. (2022) Proc. Natl. Acad. Sci. USA 119:e2110415119), we identify a binding pocket sequence motif that was present in 3D structures of sensor domains crystallised in complex with quaternary amines. Screening of sequence databases for the presence of this motif resulted in the identification of 13.000 different receptors, that formed the basis for the definition of the amine specific subfamily of dCache sensor domains (dCache_1AM). The individual sensor domain of representative receptors was generated as individual purified protein, and their analysis by thermal shift ligand screening and microcalorimetric titrations indicated that the predictions were highly precise. dCache_1AM sensor domains show a wide phylogenetic distribution in bacteria and were also detected in archaea (Fig. below). These domains were identified in a variety of receptors such as chemoreceptors, sensor histidine kinases or diguanylate/guanylate cyclases and phosphohydrolases that differ in function and mediate chemotaxis, regulate gene expression or control second messenger homeostasis, indicating the amines are central bacterial signal molecules. This article has been commented by Dlakić (2023) (Proc. Natl. Acad. Sci. USA 120:e2316830120).
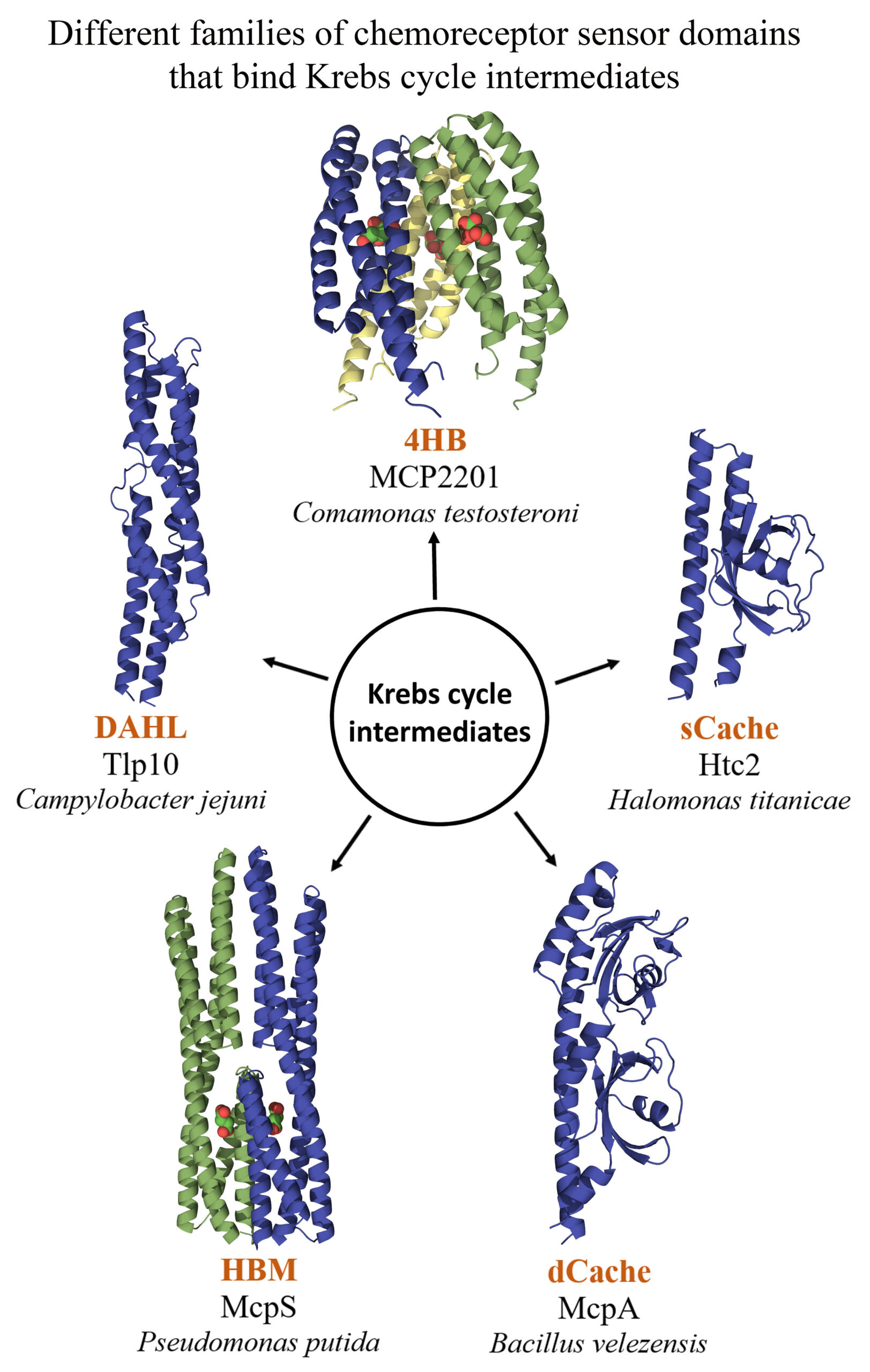
Gaining access to nutrients is the primary benefit from chemotaxis
Matilla et al. (2023) Curr. Opin. Microbiol. 75: 102358.
Our knowledge on chemoattractants, or compounds that induce a directed, flagellum-mediated movement, is growing rapidly and new chemoattractants are discovered regularly. We discuss here several observations that strongly suggest that the access to nutrients is the primary force that has driven the evolution of the chemotactic system. Among these observations are the notion that most chemoeffectors are of metabolic and that many different chemoreceptor sensor domains have evolved that bind metabolic value compounds, as illustrated in the Figure below by the different sensor domains for Krebs cycle intermediates. Furthermore, several studies report a growth-rate dependent modulation of chemotaxis gene expression and several broad ligand range chemoreceptors bind preferentially metabolic value compounds. Exchange of metabolic value compounds between bacteria and phytoplankton was shown to be enhanced by chemotaxis that in turn plays a major role in shaping marine ecosystems.
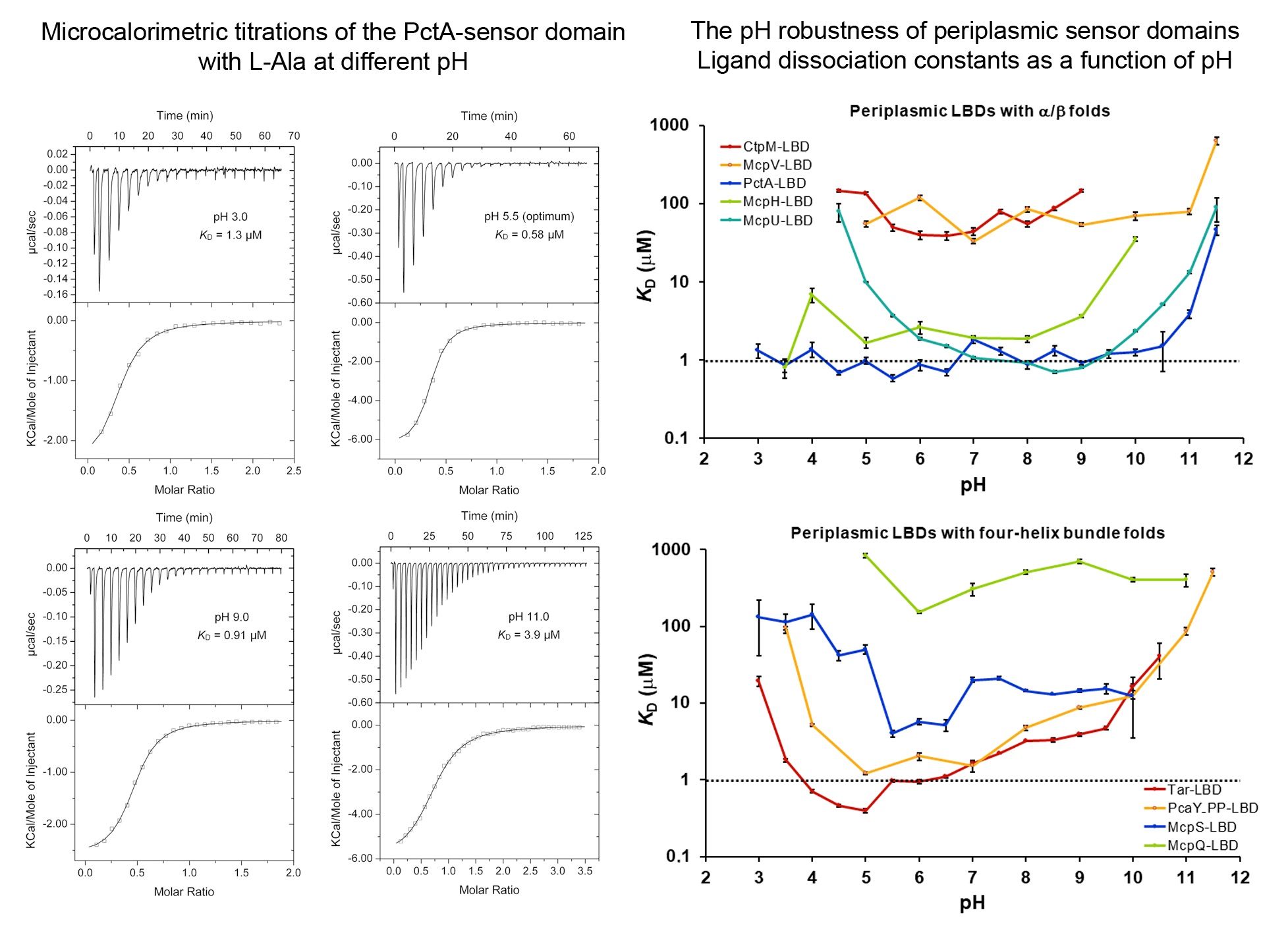
The stunning pH robustness of extracytosolic bacterial sensor domains
Signal transduction is typically initiated by the recognition of signal molecules at receptor sensor domains. Hundreds of different types of sensor domains have evolved. In this article we have investigated the capacity of members of the four major superfamilies of extracytosolic sensor domains to recognize their signals at different pH. We have furthermore analysed the pH dependence of signal recognition of periplasmic solute binding proteins as well as cytosolic sensor domains. We show that extracytosolic sensor domains possess a stunning pH robustness, which is exemplified by the sensor domains of the Tar and PctA chemoreceptors that recognized their cognate signals over 7.5 and 8.5 pH units, respectively. A similar pH robustness was observed for periplasmic solute binding proteins, whereas cytosolic sensor domains bound their ligands over a much narrower pH range. Data thus suggest that many receptors maintain their sensing capacities over a broad range permitting in turn signal integration under harsh environmental conditions. These data also permit the construction of robust biosensors.
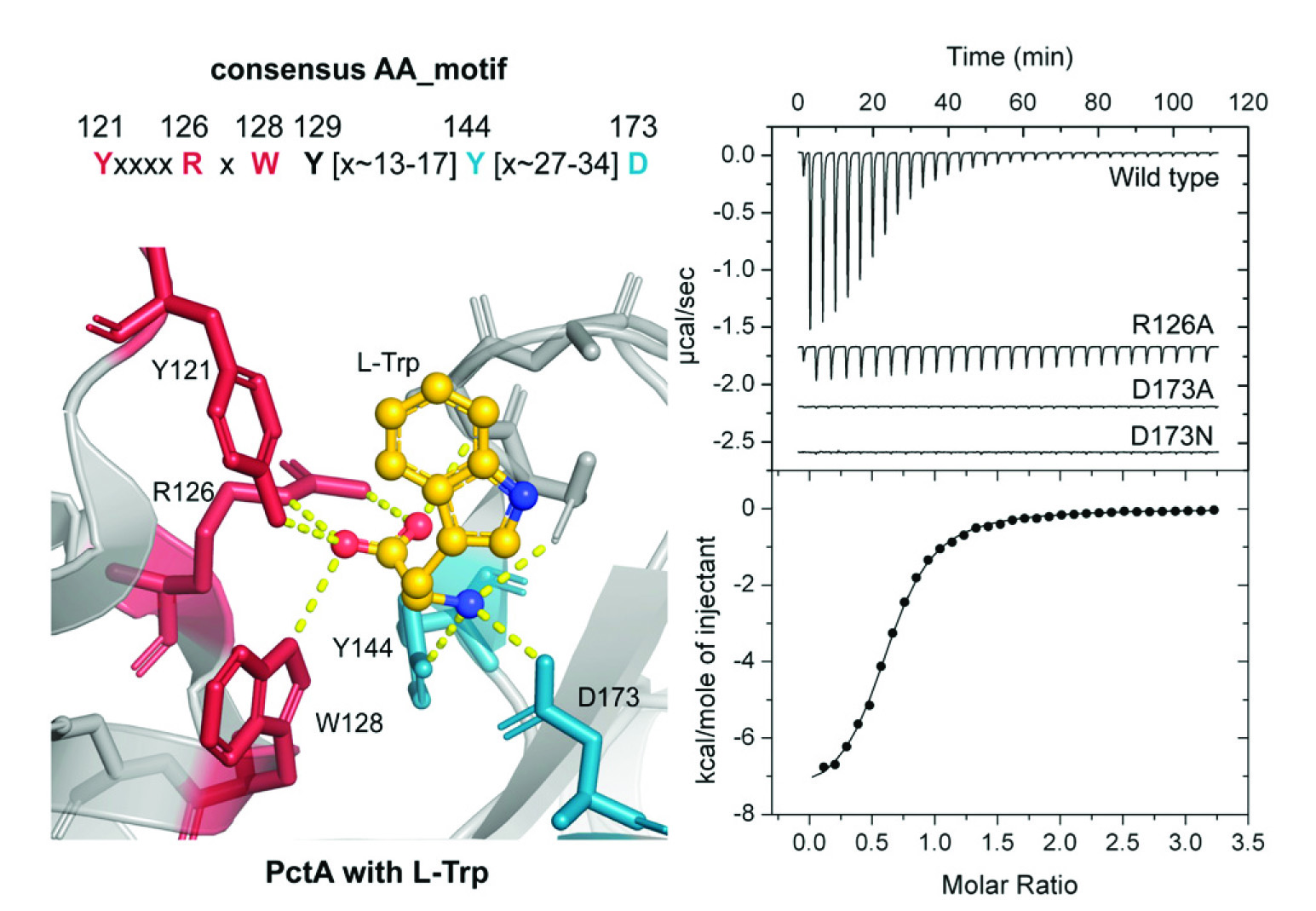
The importance of amino acid sensing for living beings
Gumerov et al. (2022) Proc. Natl. Acad. Sci. USA 119, e2110415119
Organisms throughout the Tree of Life possess the capacity to sense extracellular signals and to adjust a variety of physiological features in response. In this article we discover an extracellular sensing domain, termed dCache_1AA, that recognizes specifically amino acids. This domain is found in archaea, bacteria and different eukaryotes including the human, illustrating the importance of amino acid sensing throughout the Tree of Life. In bacteria, dCache_1AA domains are found in all major families of transmembrane signal transduction receptors including chemoreceptors, sensor histidine kinases, guanylate/adenylate cyclases, c-di-GMP phosphodiesterases or serine/threonine kinases and phosphatases, which underlines its universality. This study has also demonstrated the feasibility of predicting the ligands recognized by sensor domains taking into account the amino acids present in the ligand binding pocket.
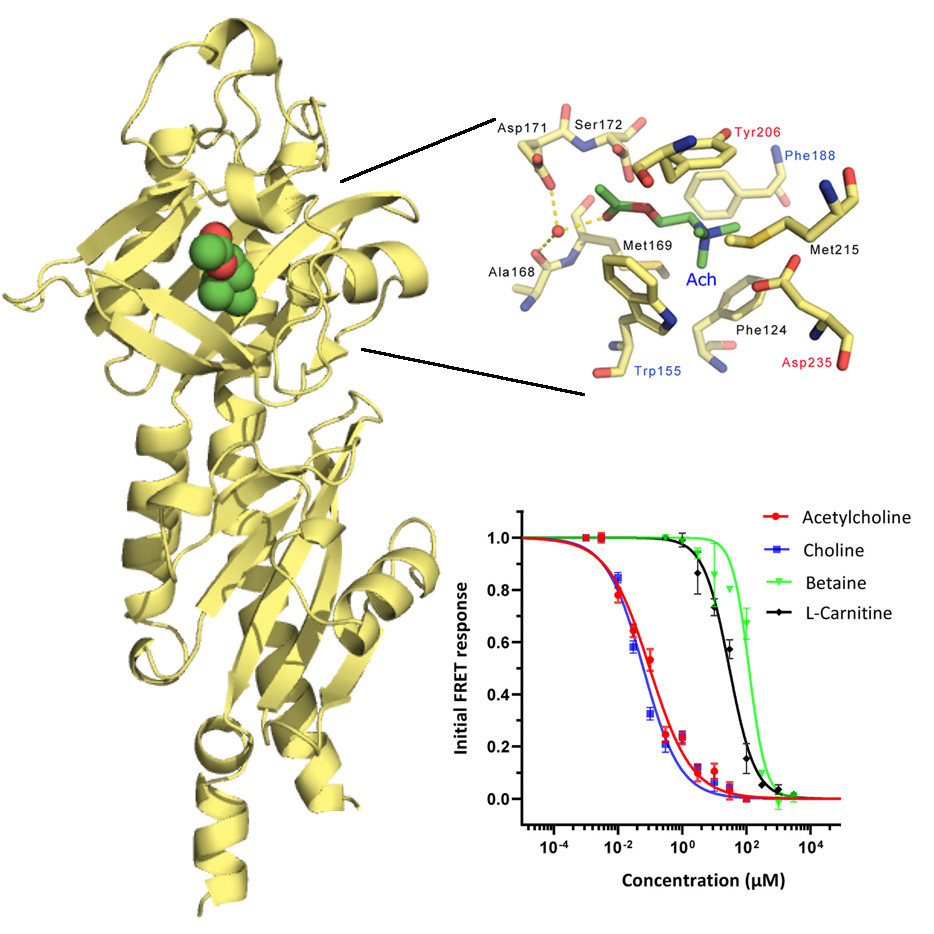
A bacterial chemoreceptor for the neurotransmitter acetylcholine
There is doubt that that the chemosensory capacity of a bacterium is a reflection of its lifestyle. However, a more detailed understanding on the chemoeffectors perceived by bacteria with a given lifestyle is still missing. In this publication we show that the human pathogen Pseudomonas aeruginosa shows strong chemoattraction towards acetylcholine, a central neurotransmitter in human. Acetylcholine was found to bind to the dCache type sensor domain of the PctD chemoreceptor. To determine the molecular determinants of acetylcholine recognition we report the high-resolution structures of the PctD sensor domain in complex with acetylcholine and that of the homologous PacA chemoreceptor that is unable to recognize acetylcholine. Interestingly, in previous studies, Reyes-Darias et al. (2015) and Corral-Lugo et al. (2018) we have identified two other P. aeruginosa chemoreceptors, PctC and TlpQ, that recognize the neurotransmitters GABA and histamine, suggesting a particular relevance of neurotransmitter chemotaxis in P. aeruginosa. This study expands the range of biologically relevant signal molecules that are perceived as chemoattractants by bacteria.
Selected publications
Cerna-Vargas, J.P., Gumerov, V.M., Krell, T., Zhulin, I.B. (2023) Amine-recognizing domain in diverse receptors from bacteria and archaea evolved from the universal amino acid sensor. Proc Natl Acad Sci USA. 120:e2305837120. (Comment article: Dlakić M. 2023. Proc Natl Acad Sci USA 120:e2316830120)
Xu, W. Cerna-Vargas, J.P., Tajuelo, A., Lozano-Montoya, A., Kivoloka, M., Krink, N., Monteagudo-Cascales, E., Matilla, M.A., Krell. T, Sourjik V. (2023) Systematic mapping of chemoreceptor specificities for Pseudomonas aeruginosa. mBio 14:e0209923.
Gavira, J.A., Rico-Jiménez, M., Ortega, Á., Petukhova, N.V., Bug, D.S., Castellví, A., Porozov, Y.B., Zhulin, I.B., Krell, T., Matilla, M.A. (2023) Emergence of an Auxin Sensing Domain in Plant-Associated Bacteria. mBio 14:e0336322.
Monteagudo-Cascales, E., Martín-Mora, D., Xu, W., Sourjik, V., Matilla, M.A. Ortega, A., Krell, T. (2022) The pH robustness of bacterial sensing. mBio e0165022.
Feng, H., Lv, Y., Krell, T., Fu, R., Liu, Y., Xu, Z., Du, W., Shen, Q., Zhang, N. Zhang, R. (2022) Signal binding at both modules of its dCache sensor domain enables the McpA chemoreceptor of Bacillus velezensis to sense different ligands. Proc. Natl. Acad. Sci USA 119:e2201747119
Gumerov, V.M., Andrianova, E.P., Matilla, M.A., Page, K.M., Monteagudo-Cascales, E. Dolphin, A.C., Krell, T. Zhulin, I.B. (2022) Amino acid sensor from bacteria to humans. Proc. Natl. Acad. Sci. USA 119: e2110415119.
Matilla, M.A., Velando, F., Tajuelo, A., Martín-Mora, D., Xu, W., Sourjik, V., Gavira, J.A., Krell, T. (2022) Chemotaxis of the human pathogen Pseudomonas aeruginosa to the neurotransmitter acetylcholine. mBio: e0345821
Matilla, M.A., Velando, F., Martín-Mora, D., Monteagudo-Cascales, E., Krell, T. (2022) A catalogue of signal molecules that interact with sensor kinases, chemoreceptors and transcriptional regulators. FEMS Microbiol Rev. 10.1093/femsre/fuab043
Matilla, M.A., Martín-Mora, D., Gavira, J.A., Krell, T. (2021) Pseudomonas aeruginosa as a model to study chemosensory pathway signaling. Microbiol. Mol. Biol. Rev. 85(1):e00151-20
Gavira JA, Gumerov VM, Rico-Jiménez M, Petukh M, Upadhyay AA, Ortega A, Matilla MA, Zhulin IB, Krell T. (2020) How Bacterial chemoreceptors evolve novel ligand specificities. mBio 11:e03066-19.
Martín-Mora, D., Ortega, A., Matilla, M.A., Martínez-Rodríguez, S., Gavira, J.A., Krell, T. (2019) The molecular mechanism of nitrate chemotaxis via direct ligand binding to the PilJ domain of McpN. mBio 10:e02334-18
Cerna-Vargas, J.P., Santamaría-Hernando, S., Matilla, M.A., Rodríguez-Herva, J.J., Daddaoua, A., Rodríguez-Palenzuela, P., Krell, T. López-Solanilla, E. (2019) Chemoperception of specific amino acids controls phytopathogenicity in P. syringae pv. Tomato. mBio 10:e01868-19.
Corral-Lugo A., Matilla M.A., Martín-Mora D., Silva Jiménez H., Mesa Torres N., Kato J., Hida A., Oku S., Conejero-Muriel M., Gavira J.A., Krell T. (2018). High-affinity chemotaxis to histamine mediated by the TlpQ chemoreceptor of the human pathogen Pseudomonas aeruginosa. mBio 9:e01894-18
Matilla M.A., Daddaoua A., Chini A., Morel B., Krell T. (2018). An auxin controls bacterial antibiotics production. Nucleic Acids Res. 46:11229-11238 46:11229-11238
Corral-Lugo A, Daddaoua A, Ortega A, Espinosa-Urgel M, Krell T. (2016) Rosmarinic acid is a homoserine lactone mimic produced by plants that activates a bacterial quorum-sensing regulator. Science Signaling 9(409):ra1.
García Fontana, C., Corral-Lugo, A., Krell, T. (2014) Specificity of the CheR2 Methyltransferase in Pseudomonas aeruginosa is Directed by C-Terminal Pentapeptides in Chemoreceptors. Science Signaling 7 (320) ra34.
Pineda-Molina, E., Reyes-Darias, J.A., Lacal, J., Ramos, J.L., García-Ruiz, J.M., Gavira, J.A., Krell, T. (2012) Evidence for chemoreceptors with bimodular ligand binding regions harboring two signal-binding sites. Proc. Acad. Natl. Sci. USA. 109, 18926-18931.
Matilla, M.A., Krell, T. (2018) The effect of bacterial chemotaxis on host infection and pathogenicity. FEMS Microbiol Rev. 42:10.1093/femsre/fux052.
Ortega, Á., Zhulin, I.B., Krell, T. (2017) Sensory Repertoire of Bacterial Chemoreceptors. Microbiol. Mol. Biol. Rev. 81: e00033-17.


